CUU LONG PHARMACEUTICAL JOINT STOCK COMPANY CONFIRMS NOT PRODUCING AND DISTRIBUTING CEPHALEXIN 500 HARD CAPSULES, BATCH NO. 04310322, NSX: 310322, HD: 310325, VD-25166-17 SAMPLED AT NGOC THANH THANH PHARMACY (GROUP 3, TAN LOI, TAN QUOI, BINH TAN, VINH LONG)
Recently, upon receiving information and requests from relevant authorities regarding the above-mentioned drug sample, Cuu Long Pharmaceutical has actively collaborated to promptly identify the results through testing and examination. Specifically:
On February 23, 2023, Cuu Long Pharmaceutical JSC received a letter No. 190/TTKN “Re: Request for Technical Standards for New Drug” addressed to Vinh Long Testing Center, No. 52, 3/2 Street, Ward 1, Vinh Long City, Vinh Long Province for Cephalexin 500 hard capsules (Cephalexin monohydrate equivalent to 500 mg anhydrous Cephalexin) with the following label information: batch no. 04310322, NSX: 310322, HD: 310325, VD-25166-17, manufactured by Cuu Long Pharmaceutical JSC (Address: No. 150, 14/9 Street, Ward 5, Vinh Long City) and sampled by Vinh Long Department of Health Inspection in collaboration with Vinh Long Testing Center at Ngoc Thanh Thanh Pharmacy (Group 3, Tan Loi, Tan Quoi, Binh Tan, Vinh Long).
On the same day, February 23, 2023, Cuu Long Pharmaceutical JSC issued a letter No. 35/CV.DCL “Re: Response to Letter No. 190/TTKN Requesting Technical Standards for New Drug”. The letter confirms that the Cephalexin 500 hard capsule sample with batch no. 04310322, NSX: 310322, HD:310325, VD-25166-17, as requested in the Vinh Long Testing Center’s letter, is not a product manufactured and distributed by Cuu Long Pharmaceutical JSC.
Vinh Long Testing Center sent the Cephalexin 500 (Cephalexin monohydrate equivalent to 500 mg anhydrous Cephalexin) sample with batch no. 04310322, NSX: 310322, HD:310325, VD-25166-17 taken from Ngoc Thanh Thanh Pharmacy (Group 3, Tan Loi, Tan Quoi, Binh Tan, Vinh Long) to Ho Chi Minh City Drug Testing Institute for quality testing. The result from the Institute is that the sample does not meet the quality requirements for qualitative criteria according to USP 43.
On March 28, 2023, a letter No. 3002/QLD-CL from the Drug Administration of Vietnam – Ministry of Health “Re: Fake Cephalexin 500” was issued. The Drug Administration concluded that based on the drug circulation permit data, there are no permitted products with the above-mentioned information.
Thus, with the information provided, it is evident that the Cephalexin 500 hard capsules (Cephalexin monohydrate equivalent to 500 mg anhydrous Cephalexin) with batch no. 04310322, NSX: 310322, HD: 310325, VD-25166-17 are not manufactured and distributed by Cuu Long Pharmaceutical JSC in the market.
To ensure the interests of customers, safety for users, and the reputation of the product quality manufactured by Cuu Long Pharmaceutical JSC, we would like to recommend customers to purchase Cephalexin 500 produced by Cuu Long Pharmaceutical JSC. We urge customers to be cautious when purchasing products with clear origins and complete legitimate invoices to ensure the products meet the quality standards registered with the authorities.
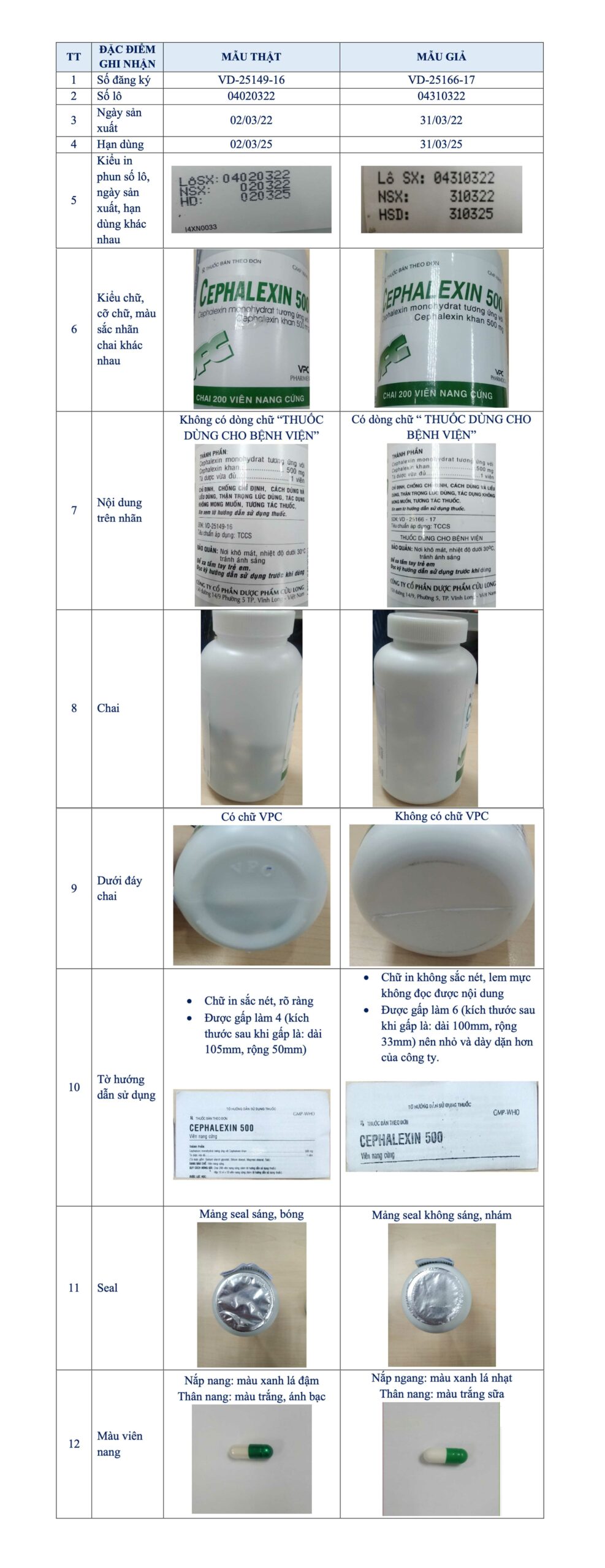
Cuu Long Pharmaceutical always expresses a firm stance and takes resolute, uncompromising actions to eliminate counterfeit and imitation activities from the market, protecting the highest interests of consumers. We are ready to cooperate with relevant authorities to clarify the matter and commit that all products manufactured by Cuu Long Pharmaceutical JSC and in circulation under the Cuu Long Pharmaceutical brand always ensure quality.